Recommendation for individuals using a screenreader: please set your punctuation settings to "most."
Descriptive Statements:
- Demonstrate knowledge of the concepts of thermal energy and temperature.
- Solve problems involving thermal expansion, specific heat, phase changes, and the first law of thermodynamics.
- Demonstrate knowledge of the kinetic theory of matter.
- Demonstrate knowledge of energy conversions, efficiency, heat transfer, and heat engines.
- Demonstrate knowledge of the second law of thermodynamics, including entropy.
Sample Item:
A closed, insulated vessel consists of a paddle wheel immersed in 0.50 kg of water at
room temperature. The shaft of the paddle wheel is attached to a motor that has an
output of 25 W. The paddle wheel rotates, churning the water.
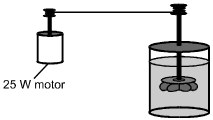
Disregarding the heat capacity of the vessel and paddle, what is the change in temperature of the water if the motor is run for 5.0 minutes?
- 0.03°C0.03 degrees Celsius
- 1.0°C1.0 degrees Celsius
- 1.8°C1.8 degrees Celsius
- 3.6°C3.6 degrees Celsius
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
D. This question requires the examinee to solve a problem related to specific heat and the first law of thermodynamics. The first law of thermodynamics states that ΔE = ΔQ − WDelta E equals Delta Q minus W, where W is the work done by the system. Since the vessel is insulated, ΔQ = 0Delta Q equals 0. Therefore, ΔE = WDelta E equals W, since work is done on the system as the paddle wheel churns the water. Therefore, cwmwΔT = (25 W)(5 min)(60 s/min)c subscript w msubscript w Delta T equals left parenthesis 25 W right parenthesis left parenthesis 5 min right parenthesis left parenthesis 60 s slash min right parenthesis. Using the value cw = 4.19 × 103 J/(kg·°C)c subscript w equals 4.19 times 10 cubed J slash left parenthesis kg dot degrees Celsius right parenthesis given in the Constants and Formulas and solving gives ΔT = 3.6°CDelta T equals 3.6 degrees Celsius.
Descriptive Statements:
- Demonstrate knowledge of the significance of the work of scientists such as Curie, Rutherford, and Planck in the development of modern physics.
- Analyze the Bohr model of the atom.
- Demonstrate knowledge of the basic principles of quantum mechanics, such as wave-particle duality and the uncertainty principle.
- Demonstrate knowledge of the basic principles of special relativity.
Sample Item:
Electrons from an electron gun pass through a double slit before striking a screen.
A counter records the number of electrons that arrive at the screen as a function
of x, the position along the screen. The distribution of the number of electrons
arriving is shown below.
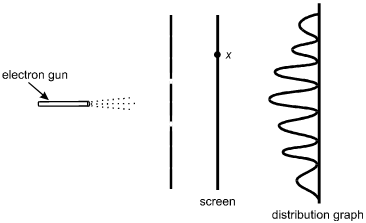
The electron gun is on the left, pointed horizontally to the right. Dotted lines represent the electrons spraying toward the right. A vertical line with two breaks, located symmetrically above and below the level of the electron gun, is between the gun and a vertical line representing the screen. A point on the screen is labeled x, somewhat above the level of the upper slit. To the right of the screen is a distribution graph with an oscillating sinusoidal wave on a vertical baseline. The peaks are to the left of the baseline. The highest peak is in the center, at the level of the electron gun, with peaks progressively lower at the upper and lower ends of the graph.
According to the principles of quantum mechanics, the distribution pattern shown is a result of:
- electrons interfering due to their wave properties.
- electrons striking the edges of the slits while passing through.
- photons interacting with each other on either side of the slits.
- electrons interacting with photons prior to arriving at the screen.
Correct Response and Explanation (Show Correct ResponseHide Correct Response)
A. This question requires the examinee to demonstrate knowledge of the wave-particle duality. In the double-slit experiment shown, the distribution of the number of electrons arriving on the screen at various positions is a result of interference. Interference is a consequence of the wave properties of electrons.
Descriptive Statements:
- Demonstrate knowledge of the structure of the nucleus, including the forces that hold it together.
- Apply knowledge of radioactive decay processes and the concept of the half-life to analyze and solve problems.
- Demonstrate knowledge of the processes of nuclear fission and nuclear fusion.
- Apply the principles of conservation of charge and mass-energy to analyze nuclear reactions.
Sample Item: